Good Manufacturing Practice (GMP)
Good Manufacturing Practice
Unlock the Future of Medicine with PharmaGroup’s GMP Solutions. Our highly qualified engineering services are tailored to the unique demands of the pharmaceutical and biotech industry, covering all relevant technical disciplines essential for the establishment and revamp of manufacturing facilities.

Key offerings
1. Quality and Compliance with GMP:
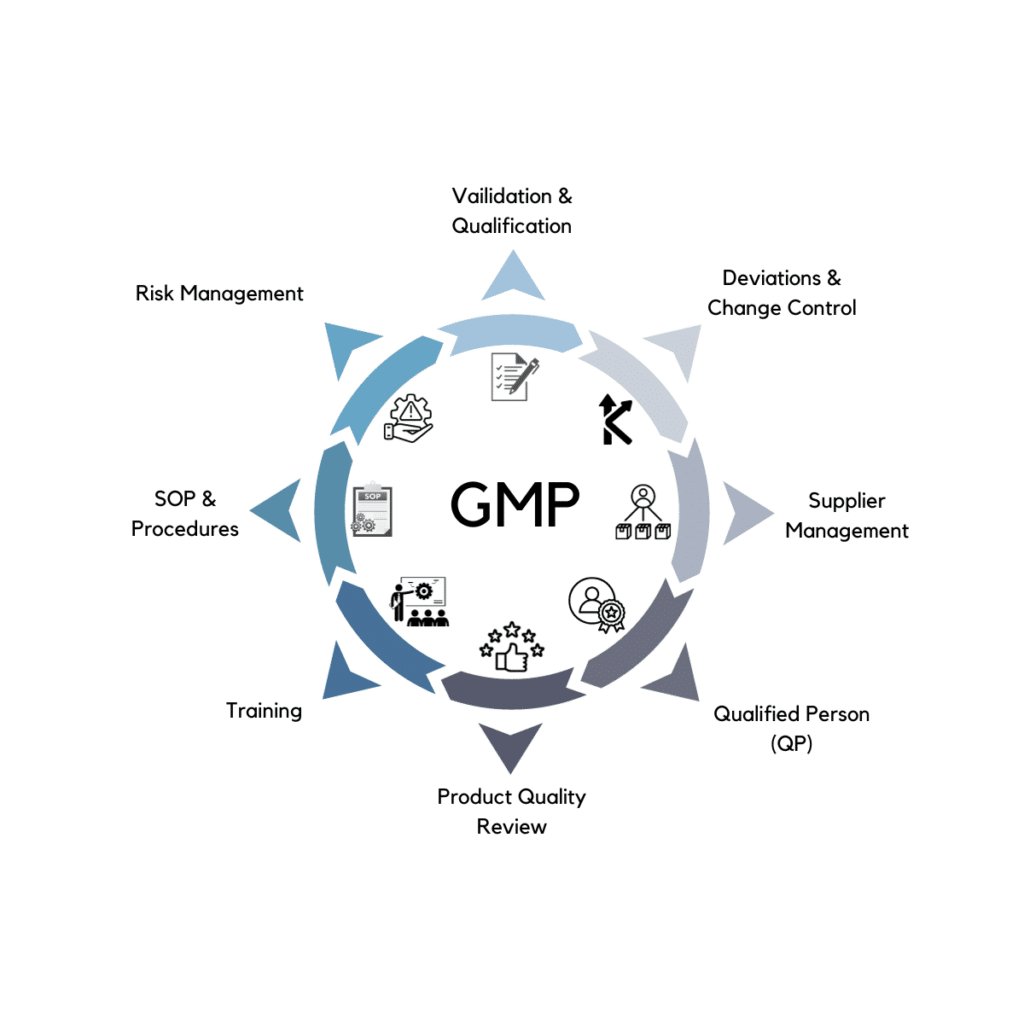
PharmaGroup’s unwavering commitment to Good Manufacturing Practice (GMP) ensures that every facet of your operations aligns with the highest quality standards. Our rigorous adherence to GMP principles guarantees the integrity of your pharmaceutical manufacturing processes.
2. Engineering Solutions:
Our skilled engineers specialize in innovative solutions, incorporating rigorous validation processes to ensure the reliability of manufacturing systems, thorough qualification procedures for equipment competence, and seamless integration of GMP principles to optimize efficiency.
3. Qualified Person and QA Personnel:
PharmaGroup supplies you with highly qualified personnel, including Qualified Persons (QPs) and QA experts. Their extensive knowledge and experience are dedicated to upholding the highest standards of quality control, compliance, and regulatory adherence.
4. Continuous Improvement:
We prioritize continuous improvement initiatives to elevate the efficiency and effectiveness of your pharmaceutical operations. Through regular assessments and feedback mechanisms, we drive ongoing enhancements, ensuring your processes remain at the forefront of industry standards.
Be at the forefront of pharmaceutical advancements with PharmaGroup. Our dedication to quality, compliance, and innovation, coupled with expertise in Validation, Qualification, Deviation Management, and Change Control, sets a new standard in pharmaceutical GMP. Contact PharmaGroup to shape the future of medicine through cutting-edge GMP solutions.

Our Services includes, but is not limited to:
- Instructions, SOPs and Quality Manual
- Training documentation for all employees
- Quality agreement with GDP service providers and contractors
- Change control and deviation management
- Customer complaints and recalls
- Qualification and oversight of GDP service providers
- Qualification of customers and suppliers
- Quality Management Review
- Audits and self-inspections
- Management of returned products
- Handling of falsified medicines
- Good Documentation Practice
- Quality Risk Management
- Shortage management
- Goods receipt and release process
Which requirements do you have to meet for GDP certification in EU?
In order to meet the requirements for a GDP license (also called Wholesaler Distribution Authorization) in an European country, a company must complie with EU GDP guidelines and local legislation. At PharmaGroup, we have specialized experts in obtaining a GDP license who can support you with the initial task as well as the maintenance of an existing license. We will help you to establish and implement all relevant processes as well as the submission of the GDP application to the local health authority and hosting of the pre-approval inspection. PharmaGroup is ready to offer you professional support and lead you through the process to obtain a GDP license and to ensure compliance.
